Resident evil mac download. Fluorine is a naturally-occurring, pale yellow-green gas with a sharp odor. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride, both white solids. Sodium fluoride dissolves easily in water, but calcium fluoride does not. Fluorine also combines with hydrogen to make hydrogen fluoride, a colorless gas.
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+, HF2^-, Fe4[Fe(CN)6]3, NH4NO3, so42-, ch3cooh, cuso4*5h2o).
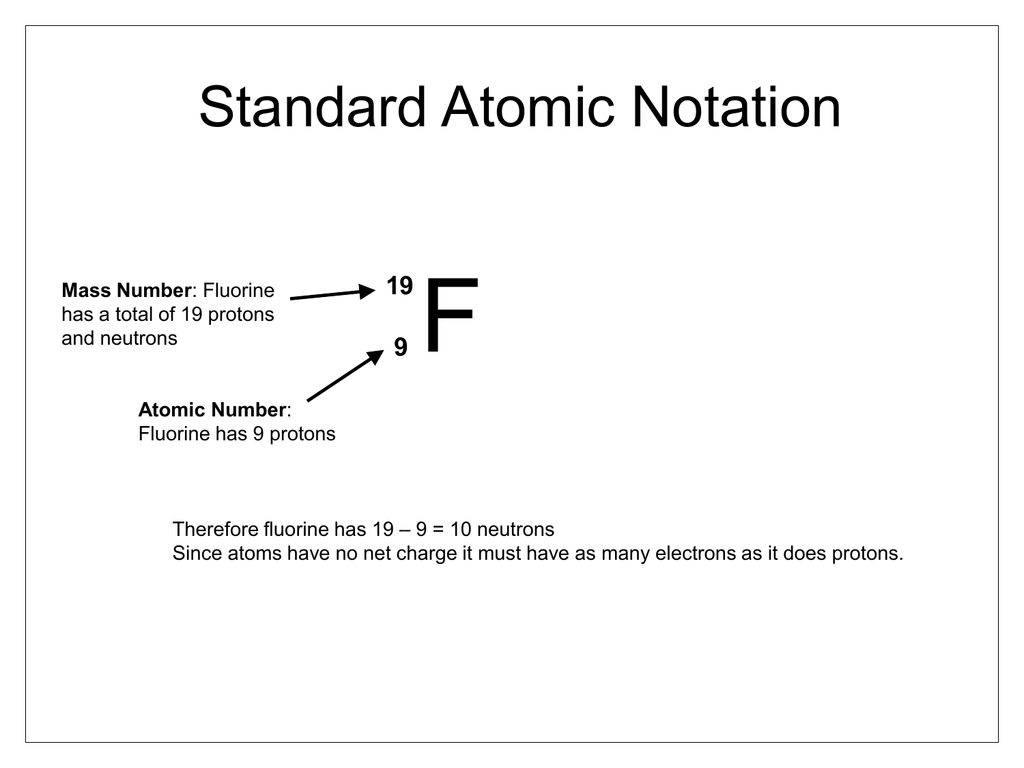
The small size of the fluorine atom makes it possible to pack a relatively large number of fluorine atoms or ions around a given coordination centre (central atom) where it forms many stable complexes—for example, hexafluorosilicate (SiF 6) 2− and hexafluoroaluminate (AlF 6) 3−. Fluorine is the most powerfully oxidizing element. Fluorine has an atomic number of 9 and mass number of 19, which means its nucleus must contain 9 protons and 10 neutrons.
Formula in Hill notation
The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Bonds between atoms of the same element (homonuclear bonds) are always divided equally.
Mass Number Of Fluorine With 8 Neutrons
When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). Unlike radicals in organic molecules, R cannot be hydrogen. Since the electrons between two carbon atoms are evenly spread, the R group does not change the oxidation number of the carbon atom it's attached to. You can find examples of usage on the Divide the redox reaction into two half-reactions page.
Rules for assigning oxidation numbers
- The oxidation number of a free element is always 0.
- The oxidation number of a monatomic ion equals the charge of the ion.
- Fluorine in compounds is always assigned an oxidation number of -1.
- The alkali metals (group I) always have an oxidation number of +1.
- The alkaline earth metals (group II) are always assigned an oxidation number of +2.
- Oxygen almost always has an oxidation number of -2, except in peroxides (H2O2) where it is -1 and in compounds with fluorine (OF2) where it is +2.
- Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.
- The algebraic sum of the oxidation numbers of elements in a compound is zero.
- The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
Assigning oxidation numbers to organic compounds

- The oxidation state of any chemically bonded carbon may be assigned by adding -1 for each bond to more electropositive atom (H, Na, Ca, B) and +1 for each bond to more electronegative atom (O, Cl, N, P), and 0 for each carbon atom bonded directly to the carbon of interest. For example:
- propene: CH3-CH=CH2
- lauric acid: CH3(CH2)10COOH
- di-tert-butyl peroxide: (CH3)3COOC(CH3)3
- diisopropyl ether: (CH3)2CH-O-CH(CH3)2
- dibenzyl sulfide: (C6H5CH2)2S
- cysteine: HO2CCH(NH2)CH2SH
These tables are based on the 2015 table with changes from the 2015 table for the values of aluminium, argon, cobalt, gold, holmium, iridium, manganese, niobium, praseodymium, protactinium, rhodium, terbium, thulium and yttrium. See report 5 June 2018. The revised value of hafnium was reported 11 December 2019
https://www.qmul.ac.uk/sbcs/iupac/AtWt/
World Wide Web version of atomic weight data originally prepared by G. P. Moss, from a file provided by D. R. Lide.
Previous values may be consulted from the 1993 table, the 1995 table, the 1997 table, the 1999 table, the 2001 table, the 2005 table, the 2007 table, the 2009 table, the 2011 table, the 2013 table or the 2015 table.
The standard atomic weights of twelve elements having two or more stable isotopes have variability of atomic-weight values in natural terrestrial materials. These are given in table 1 below. In the other lists the values quoted are those suggested for material where the origin of the sample is unknown. For radioactive elements the isotope with the longest half-life is quoted in parenthesis. The original paper should be consulted for full details of the variation in atomic weight and the half life of the radioisotopes quoted below.
A number in parentheses indicates the uncertainty in the last digit of the atomic weight.
See below for the elements listed in Atomic Number Order or Name order.
Fluorine Atomic Mass Number
See also a copy of the periodic table with atomic weights to five significant figures.
Fluorine Atomic Number And Mass Number
Table 1. List of Elements with Range of Atomic Weights.
| At No | Symbol | Name | Minimum Atomic Wt | Maximum Atomic Wt |
| 1 | H | hydrogen | 1.007 84 | 1.008 11 |
| 3 | Li | lithium | 6.938 | 6.997 |
| 5 | B | boron | 10.806 | 10.821 |
| 6 | C | carbon | 12.0096 | 12.0116 |
| 7 | N | nitrogen | 14.006 43 | 14.007 28 |
| 8 | O | oxygen | 15.999 03 | 15.999 77 |
| 12 | Mg | magnesium | 24.304 | 24.307 |
| 14 | Si | silicon | 28.084 | 28.086 |
| 16 | S | sulfur | 32.059 | 32.076 |
| 17 | Cl | chlorine | 35.446 | 35.457 |
| 18 | Ar | argon | 39.792 | 39.963 |
| 35 | Br | bromine | 79.901 | 79.907 |
| 81 | Tl | thallium | 204.382 | 204.385 |
See original paper for the range of these elements from different sources [Isotope-abundance variations and atomic weights of selected elements: 2016 (IUPAC Technical Report), Pure Appl. Chem. 2016, 88(12), 1203-1224.]
Fluorine Number Of Electrons
Table 2. List of Elements in Atomic Number Order.
How Many Neutrons Are In F 20
| At No | Symbol | Name | Atomic Wt | Notes |
| 1 | H | Hydrogen | 1.008 | 3, 5 |
| 2 | He | Helium | 4.002 602(2) | 1, 2 |
| 3 | Li | Lithium | 6.94 | 3, 5 |
| 4 | Be | Beryllium | 9.012 1831(5) | |
| 5 | B | Boron | 10.81 | 3, 5 |
| 6 | C | Carbon | 12.011 | 5 |
| 7 | N | Nitrogen | 14.007 | 5 |
| 8 | O | Oxygen | 15.999 | 5 |
| 9 | F | Fluorine | 18.998 403 163(6) | |
| 10 | Ne | Neon | 20.1797(6) | 1, 3 |
| 11 | Na | Sodium | 22.989 769 28(2) | |
| 12 | Mg | Magnesium | 24.305 | 5 |
| 13 | Al | Aluminium | 26.981 5384(3) | |
| 14 | Si | Silicon | 28.085 | 5 |
| 15 | P | Phosphorus | 30.973 761 998(5) | |
| 16 | S | Sulfur | 32.06 | 5 |
| 17 | Cl | Chlorine | 35.45 | 3, 5 |
| 18 | Ar | Argon | 39.948(1) | 1, 2, 5 |
| 19 | K | Potassium | 39.0983(1) | |
| 20 | Ca | Calcium | 40.078(4) | |
| 21 | Sc | Scandium | 44.955 908(5) | |
| 22 | Ti | Titanium | 47.867(1) | |
| 23 | V | Vanadium | 50.9415(1) | |
| 24 | Cr | Chromium | 51.9961(6) | |
| 25 | Mn | Manganese | 54.938 043(2) | |
| 26 | Fe | Iron | 55.845(2) | |
| 27 | Co | Cobalt | 58.933 194(3) | |
| 28 | Ni | Nickel | 58.6934(4) | 2 |
| 29 | Cu | Copper | 63.546(3) | 2 |
| 30 | Zn | Zinc | 65.38(2) | 2 |
| 31 | Ga | Gallium | 69.723(1) | |
| 32 | Ge | Germanium | 72.630(8) | |
| 33 | As | Arsenic | 74.921 595(6) | |
| 34 | Se | Selenium | 78.971(8) | |
| 35 | Br | Bromine | 79.904 | 5 |
| 36 | Kr | Krypton | 83.798(2) | 1, 3 |
| 37 | Rb | Rubidium | 85.4678(3) | 1 |
| 38 | Sr | Strontium | 87.62(1) | 1, 2 |
| 39 | Y | Yttrium | 88.905 84(1) | |
| 40 | Zr | Zirconium | 91.224(2) | 1 |
| 41 | Nb | Niobium | 92.906 37(1) | |
| 42 | Mo | Molybdenum | 95.95(1) | 1 |
| 43 | Tc | Technetium | [97] | 4 |
| 44 | Ru | Ruthenium | 101.07(2) | 1 |
| 45 | Rh | Rhodium | 102.905 49(2) | |
| 46 | Pd | Palladium | 106.42(1) | 1 |
| 47 | Ag | Silver | 107.8682(2) | 1 |
| 48 | Cd | Cadmium | 112.414(4) | 1 |
| 49 | In | Indium | 114.818(1) | |
| 50 | Sn | Tin | 118.710(7) | 1 |
| 51 | Sb | Antimony | 121.760(1) | 1 |
| 52 | Te | Tellurium | 127.60(3) | 1 |
| 53 | I | Iodine | 126.904 47(3) | |
| 54 | Xe | Xenon | 131.293(6) | 1, 3 |
| 55 | Cs | Caesium | 132.905 451 96(6) | |
| 56 | Ba | Barium | 137.327(7) | |
| 57 | La | Lanthanum | 138.905 47(7) | 1 |
| 58 | Ce | Cerium | 140.116(1) | 1 |
| 59 | Pr | Praseodymium | 140.907 66(1) | |
| 60 | Nd | Neodymium | 144.242(3) | 1 |
| 61 | Pm | Promethium | [145] | |
| 62 | Sm | Samarium | 150.36(2) | 1 |
| 63 | Eu | Europium | 151.964(1) | 1 |
| 64 | Gd | Gadolinium | 157.25(3) | 1 |
| 65 | Tb | Terbium | 158.925 354(8) | |
| 66 | Dy | Dysprosium | 162.500(1) | 1 |
| 67 | Ho | Holmium | 164.930 328(7) | |
| 68 | Er | Erbium | 167.259(3) | 1 |
| 69 | Tm | Thulium | 168.934 218(6) | |
| 70 | Yb | Ytterbium | 173.045(10) | 1 |
| 71 | Lu | Lutetium | 174.9668(1) | 1 |
| 72 | Hf | Hafnium | 178.486(6) | |
| 73 | Ta | Tantalum | 180.947 88(2) | |
| 74 | W | Tungsten | 183.84(1) | |
| 75 | Re | Rhenium | 186.207(1) | |
| 76 | Os | Osmium | 190.23(3) | 1 |
| 77 | Ir | Iridium | 192.217(2) | |
| 78 | Pt | Platinum | 195.084(9) | |
| 79 | Au | Gold | 196.966 570(4) | |
| 80 | Hg | Mercury | 200.592(3) | |
| 81 | Tl | Thallium | 204.38 | 5 |
| 82 | Pb | Lead | 207.2(1) | 1, 2 |
| 83 | Bi | Bismuth | 208.980 40(1) | |
| 84 | Po | Polonium | [209] | 4 |
| 85 | At | Astatine | [210] | 4 |
| 86 | Rn | Radon | [222] | 4 |
| 87 | Fr | Francium | [223] | 4 |
| 88 | Ra | Radium | [226] | 4 |
| 89 | Ac | Actinium | [227] | 4 |
| 90 | Th | Thorium | 232.0377(4) | 1, 4 |
| 91 | Pa | Protactinium | 231.035 88(1) | 4 |
| 92 | U | Uranium | 238.028 91(3) | 1, 3, 4 |
| 93 | Np | Neptunium | [237] | 4 |
| 94 | Pu | Plutonium | [244] | 4 |
| 95 | Am | Americium | [243] | 4 |
| 96 | Cm | Curium | [247] | 4 |
| 97 | Bk | Berkelium | [247] | 4 |
| 98 | Cf | Californium | [251] | 4 |
| 99 | Es | Einsteinium | [252] | 4 |
| 100 | Fm | Fermium | [257] | 4 |
| 101 | Md | Mendelevium | [258] | 4 |
| 102 | No | Nobelium | [259] | 4 |
| 103 | Lr | Lawrencium | [262] | 4 |
| 104 | Rf | Rutherfordium | [267] | 4 |
| 105 | Db | Dubnium | [270] | 4 |
| 106 | Sg | Seaborgium | [269] | 4 |
| 107 | Bh | Bohrium | [270] | 4 |
| 108 | Hs | Hassium | [270] | 4 |
| 109 | Mt | Meitnerium | [278] | 4 |
| 110 | Ds | Darmstadtium | [281] | 4 |
| 111 | Rg | Roentgenium | [281] | 4 |
| 112 | Cn | Copernicium | [285] | 4 |
| 113 | Nh | Nihonium | [286] | 4 |
| 114 | Fl | Flerovium | [289] | 4 |
| 115 | Mc | Moscovium | [289] | 4 |
| 116 | Lv | Livermorium | [293] | 4 |
| 117 | Ts | Tennessine | [293] | 4 |
| 118 | Og | Oganesson | [294] | 4 |
- Geological specimens are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty.
- Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the tabulated value should be applicable to any normal material.
- Modified isotopic compositions may be found in commercially available material because it has been subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the Table can occur.
- Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
- See table 1 for details of range and original paper for the atomic weight of the element from different sources.
Table 3. List of Elements in Name Order.
| At No | Symbol | Name | Atomic Wt | Notes |
| 89 | Ac | Actinium | [227] | 4 |
| 13 | Al | Aluminium | 26.981 5384(3) | |
| 95 | Am | Americium | [243] | 4 |
| 51 | Sb | Antimony | 121.760(1) | 1 |
| 18 | Ar | Argon | 39.948(1) | 1, 2, 5 |
| 33 | As | Arsenic | 74.921 595(6) | |
| 85 | At | Astatine | [210] | 4 |
| 56 | Ba | Barium | 137.327(7) | |
| 97 | Bk | Berkelium | [247] | 4 |
| 4 | Be | Beryllium | 9.012 1831(5) | |
| 83 | Bi | Bismuth | 208.980 40(1) | |
| 107 | Bh | Bohrium | [270] | 4 |
| 5 | B | Boron | 10.81 | 3, 5 |
| 35 | Br | Bromine | 79.904 | 5 |
| 48 | Cd | Cadmium | 112.414(4) | 1 |
| 55 | Cs | Caesium | 132.905 451 96(6) | |
| 20 | Ca | Calcium | 40.078(4) | 1 |
| 98 | Cf | Californium | [251] | 4 |
| 6 | C | Carbon | 12.011 | 5 |
| 58 | Ce | Cerium | 140.116(1) | 1 |
| 17 | Cl | Chlorine | 35.45 | 3, 5 |
| 24 | Cr | Chromium | 51.9961(6) | |
| 27 | Co | Cobalt | 58.933 194(3) | |
| 112 | Cn | Copernicium | [285] | 4 |
| 29 | Cu | Copper | 63.546(3) | 2 |
| 96 | Cm | Curium | [247] | 4 |
| 110 | Ds | Darmstadtium | [281] | 4 |
| 105 | Db | Dubnium | [270] | 4 |
| 66 | Dy | Dysprosium | 162.500(1) | 1 |
| 99 | Es | Einsteinium | [252] | 4 |
| 68 | Er | Erbium | 167.259(3) | 1 |
| 63 | Eu | Europium | 151.964(1) | 1 |
| 100 | Fm | Fermium | [257] | 4 |
| 114 | Fl | Flerovium | [289] | 4 |
| 9 | F | Fluorine | 18.998 403 163(6) | |
| 87 | Fr | Francium | [223] | 4 |
| 64 | Gd | Gadolinium | 157.25(3) | 1 |
| 31 | Ga | Gallium | 69.723(1) | |
| 32 | Ge | Germanium | 72.630(8) | |
| 79 | Au | Gold | 196.966 570(4) | |
| 72 | Hf | Hafnium | 178.486(6) | |
| 108 | Hs | Hassium | [270] | 4 |
| 2 | He | Helium | 4.002 602(2) | 1, 2 |
| 67 | Ho | Holmium | 164.930 328(7) | |
| 1 | H | Hydrogen | 1.008 | 3, 5 |
| 49 | In | Indium | 114.818(1) | |
| 53 | I | Iodine | 126.904 47(3) | |
| 77 | Ir | Iridium | 192.217(2) | |
| 26 | Fe | Iron | 55.845(2) | |
| 36 | Kr | Krypton | 83.798(2) | 1, 3 |
| 57 | La | Lanthanum | 138.905 47(7) | 1 |
| 103 | Lr | Lawrencium | [262] | 4 |
| 82 | Pb | Lead | 207.2(1) | 1, 2 |
| 3 | Li | Lithium | 6.94 | 3, 5 |
| 116 | Lv | Livermorium | [293] | 4 |
| 71 | Lu | Lutetium | 174.9668(1) | 1 |
| 12 | Mg | Magnesium | 24.305 | 5 |
| 25 | Mn | Manganese | 54.938 043(2) | |
| 109 | Mt | Meitnerium | [278] | 4 |
| 101 | Md | Mendelevium | [258] | 4 |
| 80 | Hg | Mercury | 200.592(3) | |
| 42 | Mo | Molybdenum | 95.95(1) | 1 |
| 115 | Mc | Moscovium | [289] | 4 |
| 60 | Nd | Neodymium | 144.242(3) | 1 |
| 10 | Ne | Neon | 20.1797(6) | 1, 3 |
| 93 | Np | Neptunium | [237] | 4 |
| 28 | Ni | Nickel | 58.6934(4) | |
| 113 | Nh | Nihonium | [286] | 4 |
| 41 | Nb | Niobium | 92.906 37(1) | |
| 7 | N | Nitrogen | 14.007 | 5 |
| 102 | No | Nobelium | [259] | 4 |
| 118 | Og | Oganesson | [294] | 4 |
| 76 | Os | Osmium | 190.23(3) | 1 |
| 8 | O | Oxygen | 15.999 | 5 |
| 46 | Pd | Palladium | 106.42(1) | 1 |
| 15 | P | Phosphorus | 30.973 761 998(5) | |
| 78 | Pt | Platinum | 195.084(9) | |
| 94 | Pu | Plutonium | [244] | 4 |
| 84 | Po | Polonium | [209] | 4 |
| 19 | K | Potassium | 39.0983(1) | |
| 59 | Pr | Praseodymium | 140.907 66(1) | |
| 61 | Pm | Promethium | [145] | 4 |
| 91 | Pa | Protactinium | 231.035 88(1) | 4 |
| 88 | Ra | Radium | [226] | 4 |
| 86 | Rn | Radon | [222] | 4 |
| 75 | Re | Rhenium | 186.207(1) | |
| 45 | Rh | Rhodium | 102.905 49(2) | |
| 111 | Rg | Roentgenium | [281] | 4 |
| 37 | Rb | Rubidium | 85.4678(3) | 1 |
| 44 | Ru | Ruthenium | 101.07(2) | 1 |
| 104 | Rf | Rutherfordium | [267] | 4 |
| 62 | Sm | Samarium | 150.36(2) | 1 |
| 21 | Sc | Scandium | 44.955 908(5) | |
| 106 | Sg | Seaborgium | [269] | 4 |
| 34 | Se | Selenium | 78.971(8) | |
| 14 | Si | Silicon | 28.085 | 5 |
| 47 | Ag | Silver | 107.8682(2) | 1 |
| 11 | Na | Sodium | 22.989 769 28(2) | |
| 38 | Sr | Strontium | 87.62(1) | 1, 2 |
| 16 | S | Sulfur | 32.06 | 5 |
| 73 | Ta | Tantalum | 180.947 88(2) | |
| 43 | Tc | Technetium | [97] | 4 |
| 52 | Te | Tellurium | 127.60(3) | 1 |
| 117 | Ts | Tennessine | [293] | 4 |
| 65 | Tb | Terbium | 158.925 354(8) | |
| 81 | Tl | Thallium | 204.38 | 5 |
| 90 | Th | Thorium | 232.0377(4) | 1, 4 |
| 69 | Tm | Thulium | 168.934 218(6) | |
| 50 | Sn | Tin | 118.710(7) | 1 |
| 22 | Ti | Titanium | 47.867(1) | |
| 74 | W | Tungsten | 183.84(1) | |
| 92 | U | Uranium | 238.028 91(3) | 1, 3, 4 |
| 23 | V | Vanadium | 50.9415(1) | |
| 54 | Xe | Xenon | 131.293(6) | 1, 3 |
| 70 | Yb | Ytterbium | 173.045(10) | 1 |
| 39 | Y | Yttrium | 88.905 84(1) | |
| 30 | Zn | Zinc | 65.38(2) | 2 |
| 40 | Zr | Zirconium | 91.224(2) | 1 |
- Geological specimens are known in which the element has an isotopic composition outside the limits for normal material. The difference between the atomic weight of the element in such specimens and that given in the Table may exceed the stated uncertainty.
- Range in isotopic composition of normal terrestrial material prevents a more precise value being given; the tabulated value should be applicable to any normal material.
- Modified isotopic compositions may be found in commercially available material because it has been subject to an undisclosed or inadvertant isotopic fractionation. Substantial deviations in atomic weight of the element from that given in the Table can occur.
- Element has no stable nuclides. The value enclosed in brackets, e.g. [209], indicates the mass number of the longest-lived isotope of the element. However three such elements (Th, Pa, and U) do have a characteristic terrestrial isotopic composition, and for these an atomic weight is tabulated.
- See table 1 for details of range and original paper for the atomic weight of the element from different sources.
